— Advisory panel to go over how to enhance gadget precision for clients with darker skin
by
Joyce FriedenWashington Editor, MedPage Today
January 31, 2024
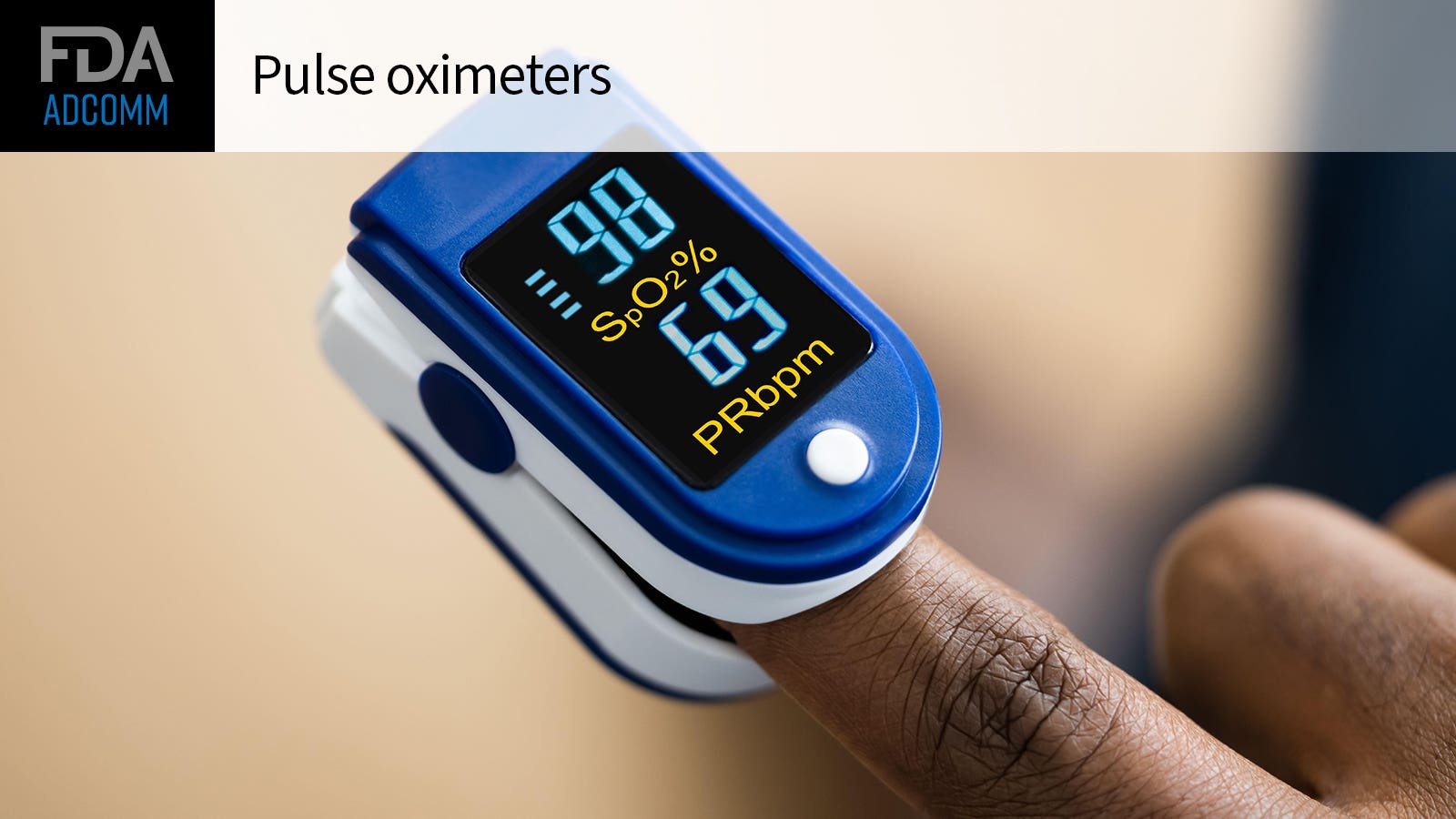
Improving the precision of pulse oximeters, specifically in clients with darker skin pigments, will be the focus at Friday’s conference of the FDA’s Anesthesiology and Respiratory Therapy Devices Panel, part of its Medical Devices Advisory Committee.
“Although pulse oximeters supply medical advantage for clients through the non-invasive evaluation of blood oxygen saturation, the threats related to incorrect pulse oximeter readings should be well comprehended,” FDA personnel composed in a instruction file for the panel.
“The COVID-19 pandemic, which led to increased usage of pulse oximeters in the medical facility and home settings, highlighted the constraints and dangers connected with this innovation, especially in clients with darker skin coloring,” they included. “Clinicians suggesting making use of pulse oximeters to clients to keep an eye on health conditions in your home, and customers utilizing OTC [over-the-counter] pulse oximeters for medical functions, need to understand these constraints to prevent hold-ups in treatment and negative client results.”
“After speaking with clients, regulators, scientists, and market, the committee will be asked to go over the proposed technique to enhance the quality of premarket research studies and associated techniques utilized to examine the efficiency of pulse oximeters for medical functions sent for premarket evaluation, thinking about a client’s skin coloring, and patient-reported race and ethnic background,” the instruction file continued. “The committee will talk about the type and quantity of information that ought to be offered by producers to FDA to examine the efficiency of pulse oximeters sent for premarket evaluation, consisting of prescription and over the counter signs, for identifying factors to consider, and methods to assist other regulative actions as required.”
The committee conference constructs on previous work the FDA has actually done on this problem. At a November 2022 conference of the very same advisory panel, members concurred that pulse oximeters are less precise in clients with darker skin, which the FDA must alert clients and service providers about the concern and advise that makers fix the disparity.
“I believe the medical proof does show diverse efficiency in clients with darker skin coloring; we saw a great deal of research studies and whatever indicated the exact same,” then-Panel Chair Steve Nathan, MD, stated at that conference. “In my mind, I believe the proof we saw was rather clear.”
The firm is including a brand-new wrinkle at this conference: FDA authorities are thinking about upgrading their 2013 assistance to pulse oximeter producers, and as part of that effort, they desire the panel to weigh in on the style of a premarket medical trial that pulse oximeter makers might carry out. The trial would be developed “to enhance the quality of premarket research studies to examine the efficiency of pulse oximeters, taking into account a client’s skin coloring, and patient-reported race and ethnic background.”
FDA personnel noted “crucial elements” of its medical trial proposition:
- Addition of a minimum of 24 individuals that cover the whole Monk Skin Tone (MST) scale. The MST has actually been verified to catch race and ethnic culture variety in colorings within the U.S. This will enhance generalizability of research study outcomes.
- A preliminary evaluation of skin coloring with MST scale followed with an unbiased coloring measurement– Individual Typology Angle– at the sensing unit website.
The firm is looking for remarks on both the technique being proposed to determine coloring, in addition to the trial size, and whether both of those components will supply adequate variety “with regard to race, ethnic culture, and skin coloring.” There are no concerns for the panel to vote on at the conference.
In the rundown file, FDA personnel summed up current research studies on the subject that they had actually discovered through a PubMed search performed in December 2023. The search utilized the technique “pulse oxim * AND (race OR racial OR pigment)” to recognize medical research studies released in between Aug. 9, 2022 and Dec. 6, 2023, “relating to the subject of pulse oximetry efficiency in people with darker skin coloring,” the rundown file kept in mind, including that extra short articles were determined by means of cross-referencing and evaluation posts.
They end up with an overall of 46 short articles: 28 from a 2022 literature evaluation and 18 brand-new ones from the current search. Of those, 7 were literature evaluations, which the personnel summed up. One 2022 evaluation of 34 potential and retrospective research studies revealed that “there is growing proof that pulse oximeters are less precise in dark-skinned people at lower saturation (<< 80%) leading to overestimations," and likewise that "a more precise technique for categorizing the research study topics into classifications by degree of skin coloring ought to be utilized in these research studies."
Another evaluation of 32 research studies concluded that “pulse oximetry might overstate blood oxygen saturation levels for individuals with dark skin in health center settings compared to gold basic SaO2 [arterial oxygen saturation] steps. The proof for the measurement predisposition recognized for other levels of skin coloring or ethnic backgrounds is more unsure.”
The Friday conference is arranged to run for 9.5 hours, consisting of a 30-minute lunch break. The FDA does not need to follow the suggestions of its advisory committees, however it typically does.
-
Joyce Frieden supervises MedPage Today’s Washington protection, consisting of stories about Congress, the White House, the Supreme Court, health care trade associations, and federal companies. She has 35 years of experience covering health policy. Follow