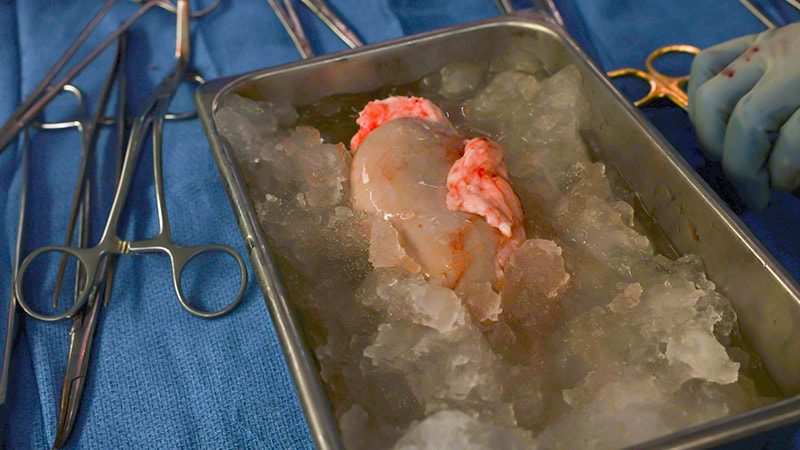
The transplant team at Massachusetts General Hospital (MGH) reports that the recipient of the first-ever transplant of a genetically edited porcine kidney into a living human was discharged from the hospital this week, 2 weeks after receiving the groundbreaking operation, and so far is doing well.
The patient experienced a cellular rejection 8 days after the transplant surgery on March 16. However, this rejection is not uncommon in conventional human kidney transplants, occurring in up to one quarter of patients within the first 3 months, and was successfully treated, Tatsuo Kawai, MD, PhD, a leader on the transplant team, told Medscape Medical News.
“The patient is off dialysis now, and he’s doing fine except that one rejection,” said Kawai, who is director of the Legorreta Center for Clinical Transplant Tolerance at MGH, in Boston, Massachusetts.
“These rejections are not uncommon in patients receiving human kidneys, so we know how to treat them,” he said.
After treatment with an antithymocyte globulin and corticosteroid, the patient’s kidney function improved and creatinine levels returned to baseline, Kawai added.
In a press statement, the patient, identified as 62-year-old Richard Slayman, of Weymouth, Massachusetts, described the ability to leave the hospital with a clean bill of health as “one of the happiest moments of my life.”
Slayman had a long history of type 2 diabetes and hypertension and had developed end-stage kidney disease (ESKD) after the failure of a previous kidney transplant from a human deceased donor in 2018 at the same center.
Engineered Porcine Model Developed Over 5 Years in Collaboration
In the 4-hour surgery, Slayman was transplanted with the kidney from a genetically engineered donor porcine model developed over 5 years in a collaboration between MGH and eGenesis, of Cambridge, Massachusetts.
As detailed in research published in 2023 in Nature, the scientists used CRISPR-Cas9 gene-editing technology to engineer the porcine model to carry 69 genomic modifications that would eliminate potentially harmful porcine cellular sugars and inactivate viruses that could cause infection in a human.
For the model, the Yucatan miniature pig breed was specifically selected as an ideal donor owing to organ sizes comparable to human organs.
Kidneys from the porcine model were initially transplanted in monkeys, and those transplants proved successful for up to 2 years.
The next experiments involved transplanting the engineered kidneys into brain-dead humans. With those also proving successful, the MGH transplant team determined that Slayman was an ideal candidate for the groundbreaking surgery, and they applied for and were granted compassionate use permission from the US Food and Drug Administration (FDA).
Immunosuppression, Infection Prevention Key Factors
The immunosuppressant regimen utilized included the investigational antibody tegoprubart, a humanized immunoglobulin G1 anti-CD40 ligand antibody, and ravulizumab, an FDA-approved monoclonal antibody.
Tegoprubart, described as an immunomodulatory cornerstone of the transplant, has previously shown success in increasing the functional life of xenograft organs — that is, organs from animals transplanted to humans.
The drug was used in the immunosuppression regimen of the second-ever pig-to-human heart transplant, performed by the University of Maryland Medical Center, Baltimore, in September 2023.
In that case, the patient died 2 months after the transplant. In contrast, the MGH surgeons noted in a press statement that “our [kidney transplant] patient is healthier than the two recipients of the pig heart transplants.”
Furthermore, “our research shows that kidneys do better than hearts,” they added.
In the first engineered pig heart transplant, the heart was later found to have been infected with a latent virus; however, no porcine-derived infectious diseases have been detected in any individuals receiving the kidney transplants, the surgical team noted.
“We remain vigilant and will meticulously monitor for any signs of infectious diseases,” they said.
Wenning Qin, PhD, the head of research at eGenesis, told Medscape Medical News that, with the encouraging survival of the monkeys for up to 2 years, “our hope is our human-compatible organ will be able to show comparable long-term survival [to that of] our preclinical nonhuman primate study.”
Meanwhile, plans for additional transplants using the porcine model are in the works, Qin said.
“We are in discussions with other hospitals to conduct other porcine transplants under FDA compassionate use authorization.”
Porcine Organ Transplants Without Need for Immunosuppression?
Looking ahead, eGenesis has a mission to develop a human-compatible organ that could allow for immunosuppression avoidance, Qin said.
“Our vision is to produce porcine donor organs that don’t require immunosuppression, [meaning] that transplant patients wouldn’t have to go on a regimen of drugs that suppresses the immune system in order to prevent rejection,” she said.
“We know that’s going to require additional engineering, but we’re going to need the human data to help inform what the engineering looks like.”
Additional Porcine Models Also Advancing
Transplant surgeon Jayme E. Locke, MD, MPH, who was involved in the earlier porcine kidney transplants in brain-dead patients, says his team is moving ahead with plans to proceed with kidney transplantation using a different engineered porcine model and immunosuppression regimen, as described in a recent paper.
Locke agrees that hopes are high for a long durability of the porcine donor organ transplant with any of the approaches.
“[The duration] has yet to be determined, but we are all hopeful that the xenografts will last the lifetime of the recipient,” Locke, who is a professor of surgery and director of the division of transplantation at the University of Alabama at Birmingham Heersink School of Medicine, in Birmingham, Alabama, told Medscape Medical News.
“We know pigs can live upwards of 30 years outside the food chain. So hopefully the organs will have a similar lifespan.”
Better Than Human Donors?
Kawai is likewise optimistic, suggesting that genetically engineered porcine kidneys could indeed represent an improvement over human-donated kidneys.
“My impression is [an engineered porcine kidney] can be better than a deceased human transplant because with deceased human kidney donors, there can sometimes be a delay in kidney function, and the patient may need dialysis because the kidney doesn’t start functioning immediately,” he said.
“In some cases, the kidney may never recover function. So, this could be better than a bad deceased human transplant.”
Longer-Term Outcomes Anticipated as ESKD Rates Rise
The United Network for Organ Sharing (UNOS) reports that the kidney is the most common organ needed for transplant, with more than 100,000 people in the US waiting for an organ for transplant and 17 people dying each day waiting for an organ.
Meanwhile, ESKD rates are estimated to increase by as much as 68% in the US by 2030.
Considering the need, the news of the porcine transplant represents “a very encouraging event for the field of transplantation,” UNOS chief medical officer David Klassen, MD, told Medscape Medical News.
“There is a lot more work to be done in terms of additional clinical trials and careful assessment of longer-term outcomes,” he cautioned.
Kawai and Klassen had no disclosures to report. Locke disclosed that his team has received grant funding from United Therapeutics. Qin is an employee of eGenesis.