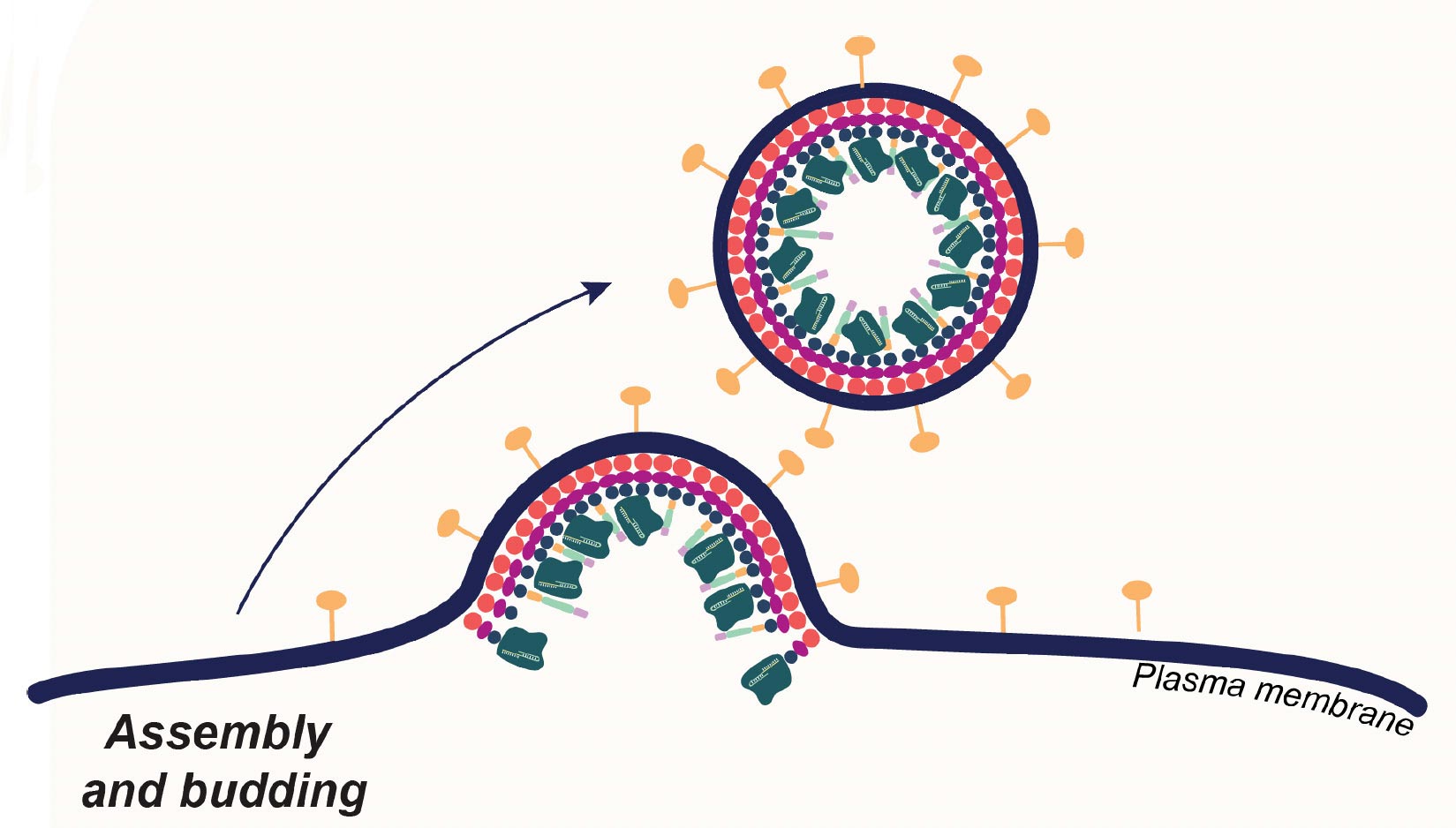
Enveloped infections get their external coat by budding from cells they’ve attacked. CRISPR-Cas9 scientists coopted this habits to produce envelope-derived lorries that encapsulate Cas9 proteins (dark green), guide RNA and transgenes. These crammed providers target and get into particular kinds of human T-cells, where they concurrently modify and place brand-new genes, turning the T-cells into cancer fighters. Credit: Jenny Hamilton, IGI/UC Berkeley
Antibody-targeted ‘enveloped shipment lorries’ selectively modify T-cells to develop CAR T-cells.
Many authorized gene treatments today, consisting of those including CRISPR-Cas9, work their magic on cells gotten rid of from the body, after which the modified cells are gone back to the client.
This strategy is perfect for targeting blood cells and is presently the technique used in recently authorized CRISPR gene treatments for blood illness like sickle cell anemia, in which modified blood cells are reinfused in clients after their bone marrow has actually been damaged by chemotherapy.
Improvement in CRISPR-Cas9 Delivery
A brand-new, precision-targeted shipment approach for CRISPR-Cas9, released on January 11 in the journal Nature Biotechnologyallows gene modifying on extremely particular subsets of cells while still in the body– an action towards a programmable shipment technique that would remove the requirement to eliminate clients’ bone marrow and body immune system before providing modified blood cells.
The shipment approach, established in the reported in Science in 2020. That treatment not just provided a transgene for a receptor targeting cancer cells, however knocked out, utilizing CRISPR, receptors not targeting the cancer.
The UC Berkeley scientists prospered in knocking out the native T-cell receptor and providing a transgene for a receptor that targeted B cells– a proxy for cancer cells. Due to the fact that the Cas9 protein was provided together with the transgene within the exact same EDV, it had a much shorter life expectancy than techniques that provide a Cas9 gene, which equates to less off-target edits.
“What we’ve attempted to attain in this paper,” Hamilton stated, “is avoiding that entire action of needing to engineer cells outside the body. We intended to systemically administer a single vector that would do both gene shipment and gene knockout in particular cell types inside the body. We utilized this shipment technique to make gene-edited CAR T-cells in vivoin the hopes that we ‘d have the ability to simplify the complicated procedure utilized to produce gene-edited CAR T-cells ex vivo“
Future Directions and Accessibility
Doudna and her laboratory continue to enhance the effectiveness of EDV-mediated shipment. Hamilton, previously a postdoctoral scientist in Doudna’s laboratory, is more establishing this shipment approach as a fellow in the IGI’s Women in Enterprising Science program. The laboratory’s supreme factor for concentrating on vectors that work in vivo is to make CRISPR treatments more broadly readily available and less expensive. In a current essay in Wired publication, Doudna described the injustices these days’s costly gene treatments, in part due to prolonged healthcare facility remains that are needed when a client goes through a bone marrow transplant.
“The treatment for sickle cell illness is forecasted to cost over $2 million per client, and just a little number of centers in the U.S. have the technological ability to supply it,” composed Doudna, who shared the 2020 Nobel Prize in Chemistry for her co-invention of CRISPR-Cas9 genome modifying. “New innovations enabling in vivo shipment of gene-editing treatments and enhanced production will be crucial to driving rates down, as will distinct collaborations in between universities, federal government and market, united with price as a typical objective. It is insufficient to merely make the tools. We need to guarantee they reach those who require them most.”
Referral: “In vivo human T cell engineering with enveloped shipment automobiles” by Jennifer R. Hamilton, Evelyn Chen, Barbara S. Perez, Cindy R. Sandoval Espinoza, Min Hyung Kang, Marena Trinidad, Wayne Ngo and Jennifer A. Doudna, 11 January 2024, Nature Biotechnology
DOI: 10.1038/ s41587-023-02085-z
In addition to Hamilton and Doudna, other co-authors of the paper are Evelyn Chen, Barbara Perez, Cindy Sandoval Espinoza, Min Hyung Kang and Marena Trinidad, all connected with the IGI and UC Berkeley’s Department of Molecular and Cell Biology, and Wayne Ngo of the Gladstone Institutes in San Francisco.
Financing was offered by the